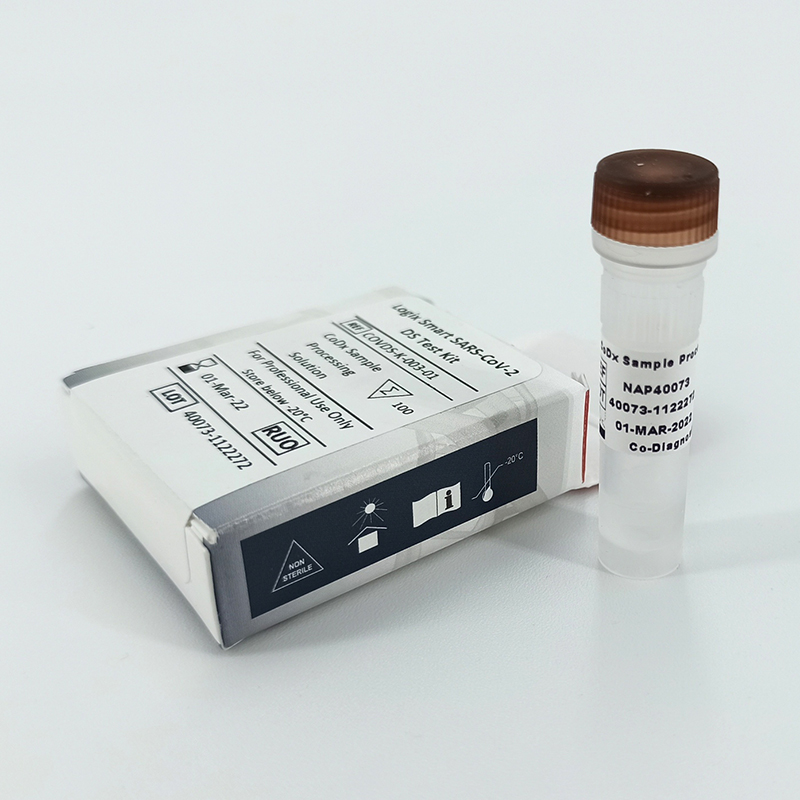
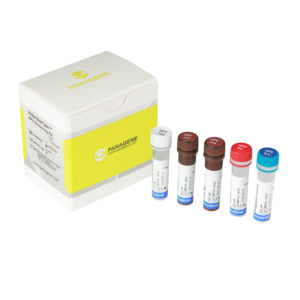
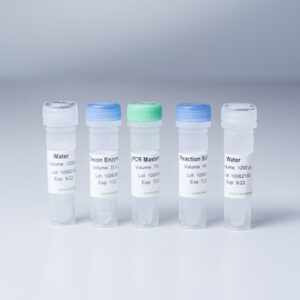
Logix Smart™ Coronavirus 2019 (COVID-19) Test Kit
REF: COVID-K-001
The Logix Smart™ Coronavirus Disease 2019 (COVID-19) Test kit is an in vitro diagnostic test that uses our patented CoPrimer™ technology for the qualitative detection of the RNA from SARS-CoV-2 coronavirus (COVID-19).
The test operates using a single step real-time real-time reverse transcriptase polymerase chain reaction (RT-PCR) process in lower respiratory tract fluids (e.g. bronchoalveolar lavage, sputum, tracheal aspirate), and upper respiratory tract fluids (e.g. nasopharyngeal and oropharyngeal swabs) from patients who meet the clinical criteria (e.g. signs and symptoms) for coronavirus disease 2019 (COVID-19) as established by WHO (WHO, 2020) and the US CDC (CDC, 2020) (e.g. fever, cough, shortness of breath, travel history to China).
Ready-to-use Master Mix, complete with RNaseP internal positive control to verify sample quality
Positive Control (PC), to verify the performance of the master mix
Nuclease-Free Water as a negative control, to verify master mix is free from contamination