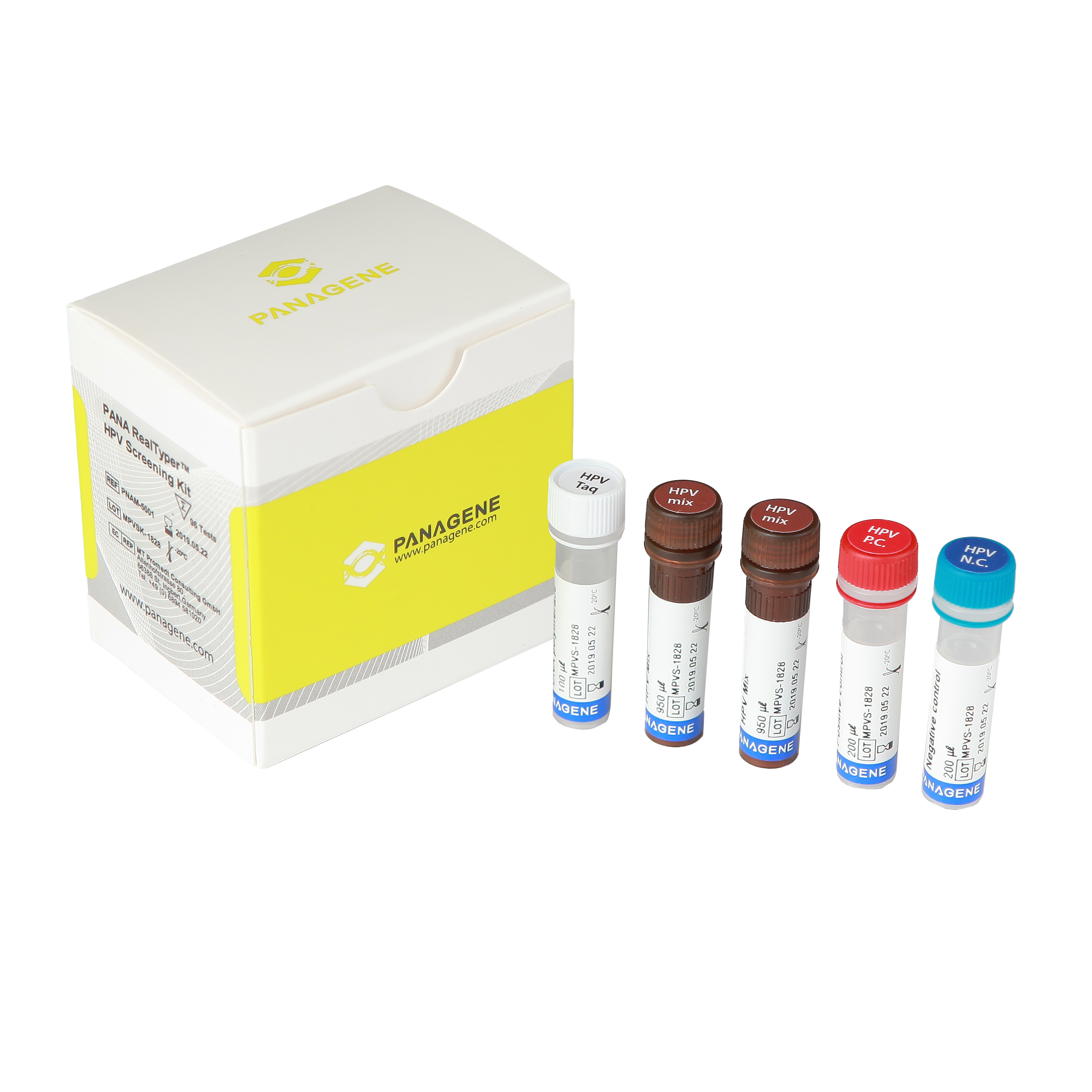
HPV is well known as the leading cause of cervical cancer. More than 40 pathogens have been found in 100 types of HPV in female genitalia. The high-risk group of these viruses is generally known to have a direct correlation with cervical cancer. That is why the WHO strongly recommends HPV DNA testing. The PANA RealTyper ™ HPV kit can detect 14 pathogens from the high-risk group and 2 pathogens from the low-risk group. With real-time PCR technology, it can quickly and accurately display test results on whether patients have those pathogens or not, and can also be used as a screening test.
The PANA RealTyper™ HPV Detection Kit is an in vitro diagnostic reagent for detecting human papillomavirus (HPV) from a cervical swab. This kit is an amplified DNA test for the qualitative detection of a total of 14 high-risk HPV types (HR) and 2 low-risk HPV types (LR) in a real-time PCR (polymerase chain reaction) system. This kit specially identifies 2 types of HR, such as 16 and 18, and 2 types of LR, such as 6 and 11, while at the same time detecting the other HPV HR types including 31, 33, 35, 39, 45, 51, 52, 56, 58, 59. , 66 y 68.